
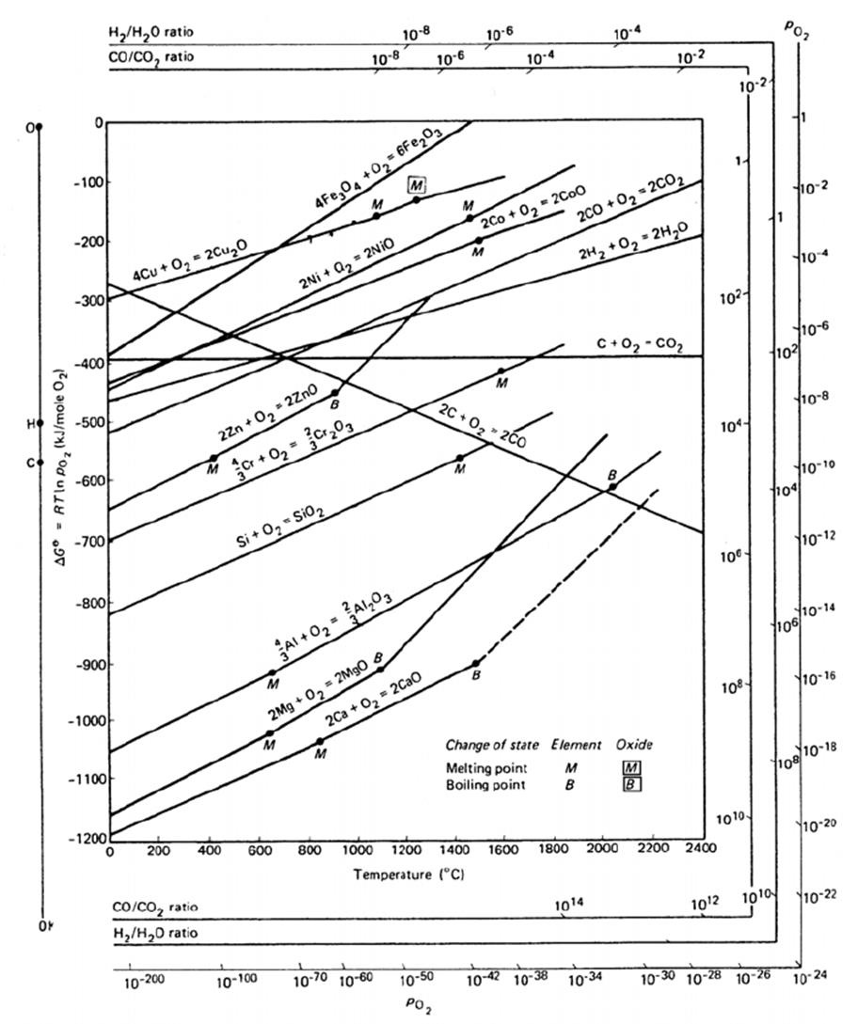

Using this property, reduction of metals may be performed as a double redox reaction at relatively low temperature. Moreover, when carbon reacts with oxygen it forms gaseous oxides carbon monoxide and carbon dioxide, therefore the dynamics of its oxidation is different from that for metals: its oxidation has a more negative ΔG with higher temperatures. Carbon is available cheaply as coal, which can be rendered to coke). In industrial processes, the reduction of metal oxides is effected using carbon. At the point of intersection the Gibbs energy is 0(zero), below this point the Gibbs energy is 0 and so, the oxides are unstable. Reduction with using a certain reductant is possible at the intersection point and higher temperatures where the ΔG line of the reductant is lower on diagram than the metallic oxide to be reduced.

#ELLINGHAM DIAGRAMS FREE#

At a sufficiently high temperature, the sign of ΔG may invert (becoming negative) and the oxide can spontaneously reduce to the metal.Īs with any chemical reaction prediction based on purely energetic grounds the reaction may or may not take place spontaneously on kinetic grounds if one or more stages in the reaction pathway have very high Activation Energies E A. In the temperature ranges commonly used, the metal and the oxide are in a condensed state (liquid or solid) with the oxygen gaseous, the reactions may be exothermic or endothermic, but the ΔG of the oxidation always becomes more negative with lower temperature, and thus the reaction becomes more probable statistically. The Ellingham diagram plots the Gibbs free energy change (ΔG) for the oxidation reaction versus the temperature. ΔG is the Gibbs Free Energy Change,ΔH is the Enthalpy Change and ΔS is the Entropy Change] Ellingham diagram for high temperature oxidationĮllingham diagrams follow from the second law of thermodynamics and are a particular graphical form of it.


 0 kommentar(er)
0 kommentar(er)
